Top Risks of Not Having a Quality Management System In Place
About LuitBiz QMS | Features | Pricing | FAQ


How Does LuitBiz QMS Help Businesses?
The 8 key-principles on which LuitBiz QMS is based are:
Customer Focus
By allowing you to pin point what your business needs to deliver the products and services fit for your customers
Strong Leadership
By ensuring that the business is aligned, well-rounded and sets goals for monitoring and evaluating performance
Involvement Of People
By creating a quality-driven culture where people openly share information and understand the company's values
Process Approach
By allowing to have full control over your SOPs and their associated checklists and forms
Increased Profits
By providing tools to identify issues before they result in rework, waste, or nonconformances
Continuous Improvement
By adhering to the four-step quality assurance method called the plan-do-check-act (PDCA) cycle
Decision Making Based On Facts
Removing subjectivity from leadership with real-time use of data to facilitate continuous improvement toward strategic goals
Continuously improve with LuitBiz QMS
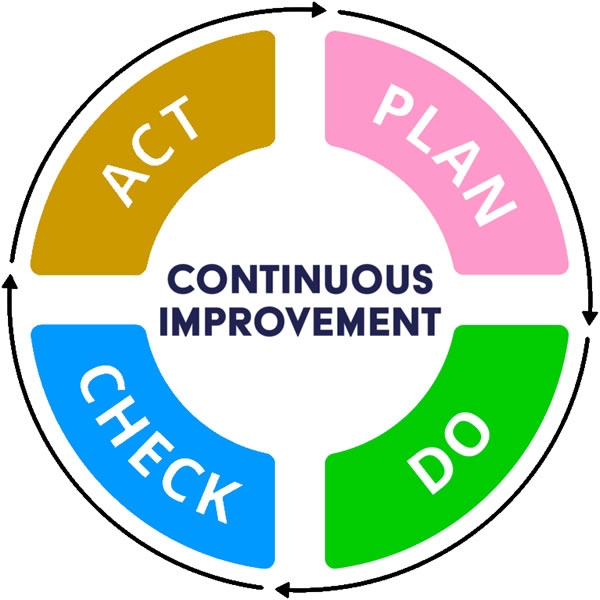
LuitBiz QMS allows you to continuously innovate and improve your products, services and processes through incremental changes over time or changes that occur all at once. Towards this end, it adopts a four-step quality assurance method called the plan-do-check-act (PDCA) cycle:
Plan Identify an opportunity for improvement and plan for change by implementing the relevant SOPs and their associated forms and checklists
Do Implement the change on a small scale, try incremental changes without adopting a big-bang approach
Check Use data to analyze the results of the change by generating the necessary reports
Act If the change was successful, implement it on a wider scale and continuously assess the results. If the change does not work, begin the cycle again.
The Quality Management Process of LuitBiz QMS involves discovering the deficiencies that exist in the production of a product, and reducing the number of defective products that get delivered to the consumer. A continuous improvement program can decrease manufacturing costs by decreasing defective product, can increase production through continuous analysis and refinement of the manufacturing process, and can find and eliminate inefficiencies throughout the organization.
Sign up for an Online Demo & Free Trial of LuitBiz QMS today
and take control of your quality documents and process forms
Manage compliances with LuitBiz QMS
In all business operations regulatory compliance has a huge impact. Companies spend a lot of time and money in meeting regulatory compliances but a single flaw in their systems can have huge repercussions and might call for huge fines. LuitBiz QMS helps organizations meet the following regulatory compliances:
FDA 21 CFR Part 11 Compliance
Required By: All drug makers, medical device manufacturers, biotech companies, biologics developers, and other FDA-regulated industries
Learn MoreFDA 21 CFR PART 510(k) & 820 Compliance
Required By: Domestic & foreign manufacturers and specification developers introducing a device to the U.S. market and repackers or relabelers of medical devices
Learn MoreGMP Compliance
Required By: All manufacturers, processors, & packagers of drugs, medical devices, some food, & blood
Learn MoreGDPR Compliance
Required By: All Companies that collect data from citizens in European Union (EU) countries will need to comply with GDPR rules for protecting customer data by May 25, 2018.
Learn MoreISO 9001 2015 Compliance
Required By: All organizations, regardless of size or industry, that want to show their ability to meet customer requirements and regulatory obligations
Learn MoreNIOSH 42 CFR Part 84 Compliance
Required By: Any company that designs, manufactures, assembles, or controls the assembly of a respirator & seeks NIOSH certification
Learn MoreOSHA Compliance
Required By: Private sector workers, workers in the state and local governments and federal government employees in USA
Learn MoreSarbanes-Oxley (SOX) Compliance
Required By: All publicly traded companies in the United States as well as wholly-owned subsidiaries and foreign companies that are publicly traded and do business in USA
Learn More